Caspase-1 (ICE) Detection
Caspase-1, originally identified as Interleukin -1β Converting Enzyme or ICE (1, 2), plays a role in processing a wide variety of proteins, most notably several cytokines (3-5) and enzymes within the glycolytic pathway (6). The creation of the inflammasome during host responses to pathogens leads to the activation of caspase-1 (7, 8) and the class of cell death known as pyroptosis. Inflammation-related disease models have illustrated a role for caspase-1 in asthma, rheumatoid arthritis, multiple sclerosis and other disorders (7, 8).
A popular method of detecting caspase-1 activation in living cell samples is the FLICA assay from ImmunoChemistry Technologies. These whole cell assays utilize fluorescent-labeled caspase-1 inhibitor probes to form covalent bonds with activated caspase-1 enzymes within living, intact cells. I have collected a short list of technical recommendations that we have shared over the years with scientists using this assay in their research. Please contact us with any questions!
 |
| Fluorescence and DIC images of cells stained with ICT's FAM-FLICA Caspase-1 Assay (FAM-YVAD-FMK) |
- Create positive controls by inducing caspase-1 with a known inducing agent.
Search publications for the best method of caspase-1 activation or pyroptosis induction in your particular cell line. For example, we have used PMA and LPS with THP-1 cells to create known positives. Contact us for more information. Create controls for each staining combination; e.g., unstained cells, FLICA-caspase-stained cells, Hoechst-nuclei-stained cells, FLICA and Hoechst-stained cells, etc. - Create negative controls.
If treating cells with an experimental reagent (e.g. pharmaceutical compounds, protein extracts, etc), include negative controls in your experiment that only receive the vehicle solvent for the experimental treatment. Create controls for each staining combination. - Consider incubation conditions and duration.While controlling all other variables, work to keep the cells "happy" during the incubation period by considering their optimal culture environment. Successful probing for active caspase-1 enzyme with FLICA (FAM-YVAD-FMK and 660-YVAD-FMK) can be accomplished with a 15-minute incubation time.
We suggest that FLICA staining occur at 37C; this may be adjusted if the cell line to be tested does not do well at this temperature. However, we advise against FLICA staining with the tubes on ice as this will greatly impact the diffusion rate and binding kinetics of the probe, and accurate staining may not occur.
In addition, many cells will not do well if kept in hypotonic solutions during incubation. The cell-permeable, non-toxic FLICA probe may be spiked directly into cell culture media for the incubation period. Cell culture media may also be used in place of the apoptosis wash buffer for the washing steps. - Perform thorough wash steps with a centrifuge and, if necessary, a centrifuge plate adaptor.
Passive washing with adherent cells is also possible by repeating the following steps 3x: aspirate the cell culture media, replace it with fresh media, and wait 10 minutes. However, some caspase-1-positive cells may have lost adherence and could be loose in the media; for this reason, the aspirated media must be collected for inclusion with the final analysis. - Read the fluorescent signal immediately, or fix and analyze within 24 hours.As the test samples contain whole, living cells, they may feasibly continue through their cell cycle or cell death process (e.g., apoptosis, pyroptosis). When the dying cell loses membrane integrity late in the process, cell components, including any labeled active caspases, will leak out; after this point, these ruptured cells would not be included in whole cell sample analysis. The FAM-FLICA Caspase-1 Assay kits (nos. 97 & 98) include a formulin-based fixative, which can easily be added 1:10 to the samples.
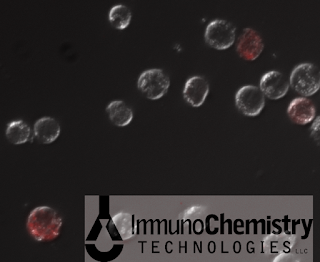 |
| Caspase-1-positive THP-1 cells fluoresce red with the inhibitor-based FLICA 660 Caspase-1 Probe (Copyright 2012 ImmunoChemistry Technologies, LLC) |
New in 2012: Far-Red FLICA™ 660 Caspase-1 Assay
- Far-red caspase-1 probe excites at 660 nm, emits at 690 nm
- Perform multiplexing studies with green-labeled biomarkers like GFP
- Whole cell assay leaves cellular system intact for accurate analysis
- Analyze via flow cytometry and fluorescence microscopy
References:
1. Black, R.A., Kronheim, S.R., Merriam, J.E., March, C.J., and Hopp, T.P. (1989) A pre-aspartate-specific protease from human leukocytes that cleaves pro-interleukin-1 beta. J. Biol. Chem. 264:5323-26.
2. Kostura, M.J. et.al. (1989) Identification of a monocyte specific pre-interleukin 1 beta convertase activity. PNAS USA 86:5227-31.
3. Ghayur, T., et.al. (1997). Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature 386:619-23.
4. Schmitz, J., et al., (2005) IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23:479-90.
5. Kumar, S. et. al. (2002) Interleukin-1F7B (IL-1H4/IL-1F7) is processed by caspase-1 and mature IL1F7B binds to the IL-18 receptor but does not induce IFN-gamma production. Cytokine 18:61-71.
6. Shao, W. et al., (2007) The caspase-1 digestome identifies the glycolysis pathway as a target during infection and septic shock. J. Biol. Chem. 282:36321-29.
7. McIntire, C. et al, (2009) Inflammasomes in infection and inflammation. Apoptosis 14:522-35.
8. Franchi, L. et al. (2009) The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nature Immunol. 10:241-47.
©2012 ImmunoChemistry Technologies, LLC. All Rights Reserved. | Bright Minds, Bright Solutions.
No comments:
Post a Comment